O 2 co 2 c. Co o 2 co 2 b.
 Chem 11
Chem 11 How many grams of kcl will be formed from 273 g of kclo 3.
Chemistry stoichiometry worksheet. Balancing equations and simple stoichiometry. 2 kclo 3 2 kcl 3 o 2 a. Chemical reactions and stoichiometry.
Chapter 6 balancing and stoichiometry worksheet and key topics. These are used in chemistry to solve stoichiometry problems with ease and understanding. What are the reactants.
Balance the following chemical reactions. Stoichiometry worksheet 1 answers 1. Learn for free about math art computer programming economics physics chemistry biology medicine finance history and more.
Sample molarity stoichiometry worksheet. 4li s o 2 g 2li 2o s a. Given the following equation.
More fun for the whole chemist family. Just what it sounds like. What is the product.
2 c 4h 10 13 o 2 8 co 2 10 h 2o show what the following molar ratios should be. Croh 3 hclo 4 crclo 4 3 h 2 o write the balanced chemical equations of each reaction. How many moles of o 2 can be produced by.
Balancing equations writing a chemical equation stoichiometry practice. Using molarity and stoichiometry. C 4h 10 h 2o 2.
Ch 3 nh 2 o 2 co 2 h 2 o n 2 hint f. How many grams of kclo 3 are needed to make 300 grams of kcl. Kno 3 kno 2 o 2 c.
C 4h 10 co 2 e. How many grams of fe 2o 3 are produced when 427 grams of fe is reacted. One of the most important ideas in chemistry is the mole concept.
What does the s after the formula of lithium oxide signify. 2 kclo 3 e 2 kcl 3 o 2 10. C 4h 10 o 2 b.
Khan academy is a nonprofit with the mission of providing a free world class education for anyone anywhere. 4 fe 3 o 2 e 2 fe 2o 3 13. 12 g 12 c exactly mole 12 c atoms.
You may also see sample atomic structure worksheets. O 3 o 2 d. Chemical reactions and stoichiometry.
More exciting stoichiometry problems. How many grams of fe 2o 3 are produced when 170 grams of o. How many grams of o 2 will be formed from 376 grams of kclo 3.
Summary notes revision videos and past exam questions by topic for cie igcse chemistry topic 4 stoichiometry. I love the smell of stoichiometry in the morning. O 2 h 2o d.
A mole of substance is that amount that contains as many elementary units atoms molecules or formula units depending on the nature of the substance as there are atoms in exactly 12 grams of an isotopically pure sample of 12 c. Nh 4 no 3 n 2 o h 2 o e. Stoichiometry using molarity worksheet.
This worksheet contains optional answers and short answers that are to be filled up in the blanks. Given the following equation. The most fun you can have with a calculator.
 Stoichiometry Worksheet Equation Chemical Reaction Chemistry Png
Stoichiometry Worksheet Equation Chemical Reaction Chemistry Png  Stoichiometry Crossword Puzzle Mole Ratios Worksheet 2 In 1 Chemistry Bundle
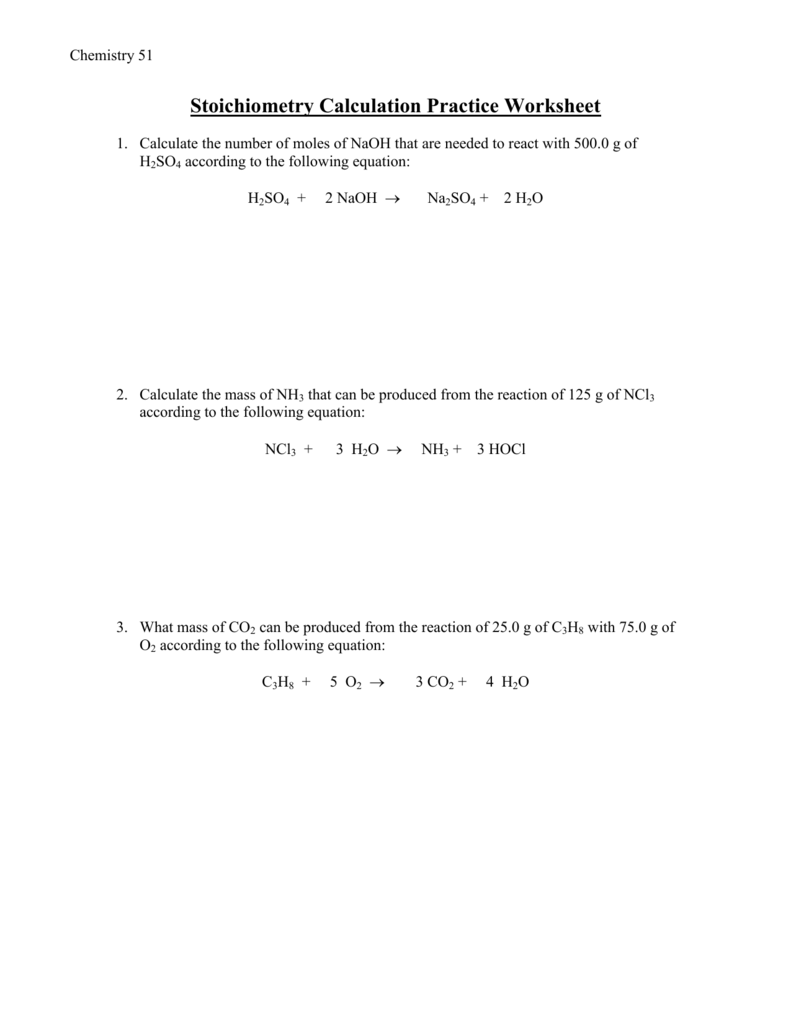
Stoichiometry Crossword Puzzle Mole Ratios Worksheet 2 In 1 Chemistry Bundle  Stoichiometry Calculation Practice Worksheet
Stoichiometry Calculation Practice Worksheet  Chm 113l Rs W3 Stoichiometry Chm 113 Chemistry Studocu
Chm 113l Rs W3 Stoichiometry Chm 113 Chemistry Studocu  Pictures Chemistry Stoichiometry Worksheet Getadating Chemical
Pictures Chemistry Stoichiometry Worksheet Getadating Chemical  Chemistry Ii Worksheet Stoichiometry Review 1 Complete And
Chemistry Ii Worksheet Stoichiometry Review 1 Complete And  Cp Chemistry Name Stoichiometry Iii Ws Period Solve The
Cp Chemistry Name Stoichiometry Iii Ws Period Solve The  Balancing Equations Practice Worksheet Answer Key Awesome Balancing
Balancing Equations Practice Worksheet Answer Key Awesome Balancing  Fun Chemistry Activity Intro To Stoichiometry Science High
Fun Chemistry Activity Intro To Stoichiometry Science High 

0 comments